Background: The discovery of IDH1 and IDH2 mutations in AML, and the accompanying functional implications of the resulting neomorphic activity of these mutated enzymes, has resulted in FDA approved targeted therapies. Similarly, the changes brought about in methylation due to IDH mutations in the AML genome have ushered in treatment regimens of venetoclax with hypomethylating agents. In this setting, where traditional chemotherapy regimens also are applied for treatment of primary diagnoses, the available outcomes data for all three therapeutic approaches should now provide clear metrics of predicted response. However, given a lack of direct comparison studies, no guidance exists as to which treatment choice will provide best response in IDH-mutated patients. Furthermore, emerging data about the importance of the biologic context on the response to different agents, including co-existing gene mutations and patient age, pose additional questions that need to be systematically addressed in order to provide patients with the best treatment.
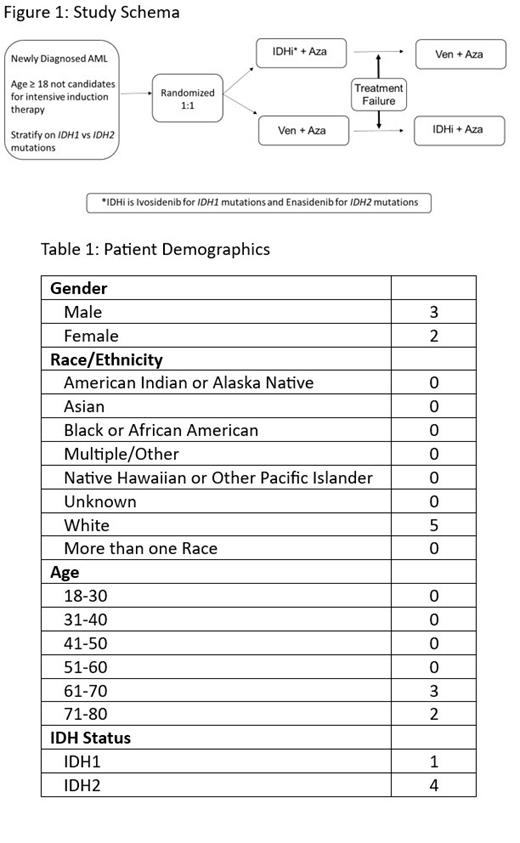
Study Design and Methods: This is a randomized, multi-site Phase 2 study (NCT05401097) comparing overall treatment failure rates at 24 months between two sequences of first-line and second-line therapies in patients ≥ 18 years with newly diagnosed IDH1/2 mutated AML who are not candidates for intensive induction therapy (Figure 1). Patients are randomized into one of the two arms in a 1:1 ratio with stratification based on the type of IDH mutation. Randomized patients are then assigned to receive treatment sequence A (IDHi+Aza followed by ven+aza) or treatment sequence B (ven+aza followed by IDHi+aza). Five patients have been randomized and enrolled on study to date.
Patients randomized to first-line IDHi will receive Ivosidenib 500mg po orally daily on Days 1-28 ( IDH1) or Enasidenib 100mg po orally daily on Days 1-28 ( IDH2), each of 28-day cycle. Aza will be given at 75mg/m 2 daily on days 1-7 or days 1-5/8-9 of each 28-day cycle. Patients can receive a maximum of 5 cycles to attain CR/CRh/CRi. Patients who achieve CR/CRh/CRi will continue IDHi+aza until unacceptable toxicity, withdrawal of consent for further protocol treatment, relapse, or death (henceforth referred to as 'cessation'). If a patient does not meet these parameters, progresses during the first 5 cycles, does not attain CR/CRh/CRi by the end of 5 cycles, attains CR/CRh/CRi by the end of 5 cycles and subsequently relapses, or has unacceptable toxicity, then the patient will stop therapy with IDHi+aza. For patients on second line Ven+aza, they may receive a maximum of 3 cycle of Ven+aza to achieve CR/CRh/CRi. Patients who achieve CR/CRh/CRi will continue Ven+aza until 'cessation.' If a patient progresses during the first 3 cycles, does not attain CR/CRh/CRi by the end of 3 cycles, attains CR/CRh/CRi by the end of 3 cycles and subsequently relapses, or has unacceptable toxicity, they will then come off study treatment.
Patients randomized to first-line Ven+aza will receive ven dosing per the FDA-label. Aza will be given at 75mg/m 2 daily on days 1-7 of each 28-day cycle. Patients can receive a maximum of 3 cycles to attain CR/CRh/CRi. Patients who achieve CR/CRh/CRi will continue Ven+aza therapy until 'cessation.' If a patient progresses during the first 3 cycles, does not attain CR/CRh/CRi by the end of 3 cycles, attains CR/CRh/CRi by the end of 3 cycles and subsequently relapses, or has unacceptable toxicity, then the patient will stop therapy with the Ven+aza. For patients on second line IDHi+aza, they may receive a maximum of 5 cycle of IDHi+aza to achieve CR/CRh/CRi. Patients who achieve CR/CRh/CRi will continue IDHi+aza until 'cessation.' If a patient progresses during the first 5 cycles, does not attain CR/CRh/CRi by the end of 5 cycles, attains CR/CRh/CRi by the end of 5 cycles and subsequently relapses, or has unacceptable toxicity, they will then come off study treatment. Longitudinally embedded, comprehensive correlative studies will provide integrated genomic profiling of the leukemic clone during different disease stages and assess for potential resistance mutations or clonal evolution that may be predictors of relapse in this difficult to treat patient population. In addition, ultra-sensitive measurable-residual disease testing (ctDNA) and single-cell based combined transcriptional and mutational profiling will complement the detailed assessment.
Disclosures
Madanat:GERON: Consultancy; Sierra Oncology: Honoraria; Morphosys: Honoraria, Other: travel reimbursement; Stemline therapeutics: Honoraria; Taiho oncology: Honoraria; Novartis: Honoraria; OncLive: Honoraria; MD Education: Honoraria; Rigel Pharmaceuticals: Honoraria; Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement. Stein:Abbvie: Consultancy; Neoleukin: Consultancy; Gilead: Consultancy; Syndax: Consultancy; OnCusp: Consultancy; CTI Biopharma: Consultancy; Foghorn: Consultancy; Bristol Myers Squib: Consultancy, Research Funding; Eisai: Research Funding; Novartis: Consultancy; Janssen: Consultancy; Agios: Consultancy; Jazz: Consultancy; Genesis: Consultancy; Genentech: Consultancy; Menarini: Consultancy; Servier: Consultancy; Calithera: Consultancy; Daiichi: Consultancy; Aptose: Consultancy; Syros: Consultancy; Astellas: Consultancy; Ono Pharma: Consultancy; Blueprint: Consultancy; PinotBio: Consultancy. Zeidner:Daiichi Sankyo: Honoraria; Astex: Research Funding; Arog: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Immunogen: Honoraria; Servier: Consultancy, Honoraria; Shattuck Labs: Honoraria, Research Funding; Stemline: Research Funding; Sumitomo Dainippon Pharma: Research Funding; Takeda: Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Foghorn: Consultancy; Jazz: Research Funding; Merck: Research Funding; Novartis: Consultancy; Sellas: Consultancy. Eisfeld:Karyopharm Therapeutics: Other: spouse employment; Astra Zeneca: Honoraria, Other: CEI Advisory Board; OncLive: Honoraria. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal